TransplantTrace Chimerism
Your chimerism monitoring. Leveled up.
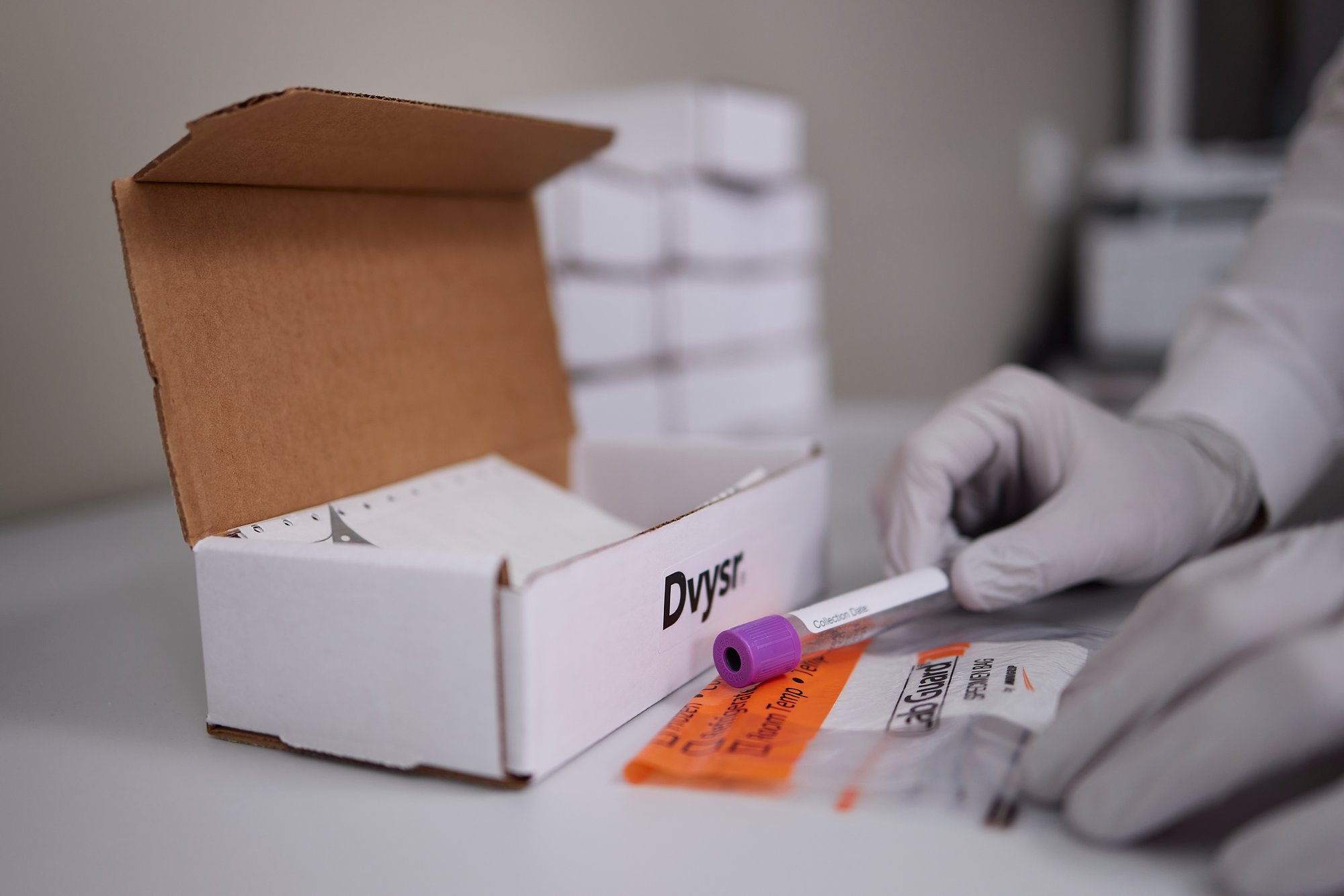
NGS-based chimerism analysis
NGS-based testing provides higher sensitivity, accuracy, and precision across a wide dynamic range versus short tandem repeat (STR) and real-time PCR-based testing methods for assessment of chimerism1. NGS-based analysis overcomes the current challenges of standard chimerism monitoring methods by combining the accuracy of STR at high chimerism levels, and the sensitivity of real-time PCR, in a single method.

Benefits of TransplantTrace Chimerism
Nearly 23,000 hematopoietic stem cell transplantations (HSCT) are performed in the US annually, of which nearly a third are allogeneic transplants, where stem cells are taken from a donor2. HSCT is a curative treatment for a range of malignant hematologic diseases. Chimerism monitoring is generally performed at 30, 90, and 180 days post-transplantation3. Research has shown that more frequent monitoring, weekly or bi-weekly, for chimerism during the first 6 months post-transplant may be important in improving patient outcomes, for some conditions, such as acute leukaemia4.
Methods with high precision and sensitivity, such as NGS-based testing, enable early detection of microchimerism changes. NGS technology allow for one method to be used irrespective of the level of mixed chimerism.
Detect chimerism as early as possible
Our highly sensitive TransplantTrace Chimerism service can detect down to 0.05% chimerism, allowing for monitoring of microchimerism. Studies have shown that increased amounts of recipient DNA need to be monitored at very low levels, as increases in microchimerism (chimerism below 1%) following HSCT can be indicative of relapse or rejection5. The most widely used method of chimerism monitoring, STR-based methods limits detection to 1-5% chimerism, and therefore has difficulty detecting microchimerism. Early detection of increasing chimerism provides physicians with the possibility of appropriate and timely therapy to improve the patient outcome6.
Data you can trust
TransplantTrace Chimerism results are provided in a straightforward and easily accessible report, giving physician the data they need in order to best manage their patients. We offer tailored services to our clients where we can present data according to physicians need: present a calculation of chimerism, and visualize chimerism monitoring over time. Our report provides physicians with a simplified and robust overview of all the key information needed for each patient, to provide best care possible.
Non-invasive
Current sampling for post-transplantation monitoring can be taken from the bone marrow directly, or peripheral blood. Research does not currently show a greater predictive benefit of bone marrow sampling versus peripheral blood7. As highlighted previously, more frequent sampling has the potential to improve the predictive value of microchimerism. However, bone marrow sampling is invasive and uncomfortable for patients, and therefore more frequent sampling, and the benefits it confers, is feasible by using peripheral blood, the validated specimen type for this LDT8. TransplantTrace Chimerism only requires a peripheral blood sample to obtain an accurate and sensitive result.
Why choose an NGS-based service over STR and qPCR?
STR analysis for chimerism monitoring is the most widely utilized method currently. STR and NGS both have high accuracy and linearity at chimerism greater than 1%, as seen in the figure.
The most widely used method of chimerism monitoring, STR-based techniques, can only detect down to 1-5% chimerism, and therefore cannot detect microchimerism, which is defined as chimerism below 1%. STR methods are insufficient in providing detection of microchimersm, to allow physicians to instigate meaningful interventions9. Therefore, a more sensitive method is needed to monitor microchimerism.
qPCR has emerged as an additional method for more accurately monitoring lower levels of chimerism. The figure also shows that qPCR and NGS methods have good linearity at chimerism levels under 1%. However, qPCR becomes inaccurate above 20% of chimerism. Therefore, to accurately cover a wide dynamic range of chimerism, a service using both STR and qPCR would be needed.
The figure shows that TransplantTrace Chimerism provides accurate results across the entire dynamic range of chimerism, and therefore only one method is needed.

%20(720%20x%20720%20px)%20(400%20x%20400%20px)%20(720%20x%20720%20px).png?width=720&height=720&name=Namnl%C3%B6s%20(1200%20x%20628%20px)%20(720%20x%20720%20px)%20(400%20x%20400%20px)%20(720%20x%20720%20px).png)
.jpg)
Enhanced customer satisfaction
Contact our team to sign up your patients for testing. Devyser Genomic Laboratories offers an optimal sample collection, testing, and reporting experience. We are here to help at every stage, so get in touch.
Contact order@us.devyser.com to order.
How to order our test
STEP 1
Place order for free sample collection kit at order@us.devyser.com
STEP 2
Download and complete the test requisition form.
STEP 3
Ship sample to Devyser Genomic Laboratories
STEP 4
Results sent to physician
STEP 5
Devyser Genomic Laboratories bills the patient directly
Frequently Asked Questions (FAQs)
What specimen is required for the test?


The test requires a whole blood sample in a single EDTA tube.
When can I expect results?


You will receive results within 10 business days from us receiving the sample.
How does a patient pay?


Patients are billed directly by us following the sending of results.
Is your laboratory CLIA-certified?


Yes, our laboratory is CLIA-certified (CLIA number 11D2278668) and CAP-accreditation pending.
References
1. Brow D, Shike H, Kendrick J, et al. Assessment of chimerism by next generation sequencing: A comparison to STR/qPCR methods. Hum Immunol. Published online March 28, 2024. doi:10.1016/j.humimm.
2. D'Souza A, Fretham C, Lee SJ, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States [published correction appears in Transplant Cell Ther. 2022 Oct;28(10):715-716]. Biol Blood Marrow Transplant. 2020;26(8):e177-e182. doi:10.1016/j.bbmt.
3. Blouin AG, Ye F, Williams J, Askar M. A practical guide to chimerism analysis: Review of the literature and testing practices worldwide. Hum Immunol. 2021;82(11):838-849. doi:10.1016/j.humimm.
4. Haugaard AK, Kofoed J, Masmas TN, et al. Is microchimerism a sign of imminent disease recurrence after allogeneic hematopoietic stem cell transplantation? A systematic review of the literature. Blood Rev. 2020;44:100673. doi:10.1016/j.blre.
5.Tang X, Alatrash G, Ning J, et al. Increasing chimerism after allogeneic stem cell transplantation is associated with longer survival time. Biol Blood Marrow Transplant. 2014;20(8):1139-1144. doi:10.1016/j.bbmt.
6. Zeiser R, Spyridonidis A, Wäsch R, et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2005;19(5):814-821. doi:10.1038/sj.leu.2403719
7.Gambacorta V, Parolini R, Xue E, et al. Quantitative PCR-based chimerism in bone marrow or peripheral blood to predict acute myeloid leukemia relapse in high-risk patients: results from the KIM-PB prospective study. Haematologica. 2020;106(5):1480-1483. Published 2020 Sep 10. doi:10.3324/haematol.2019.238543
8. Navarro-Bailón A, Carbonell D, Escudero A, et al. Short Tandem Repeats (STRs) as Biomarkers for the Quantitative Follow-Up of Chimerism after Stem Cell Transplantation: Methodological Considerations and Clinical Application. Genes (Basel). 2020;11(9):993. Published 2020 Aug 25. doi:10.3390/genes11090993
9. Stahl T, Rothe C, Böhme MU, Kohl A, Kröger N, Fehse B. Digital PCR Panel for Sensitive Hematopoietic Chimerism Quantification after Allogeneic Stem Cell Transplantation. Int J Mol Sci. 2016;17(9):1515. Published 2016 Sep 9. doi:10.3390/ijms17091515



